CDCが新しく発表した報告書によると、Pfizerのワクチンはコロナ感染の合併症としての小児多系統炎症性症候群( MIS-C )を91%防ぐ効果があるとのこと。https://t.co/JgTWz8uwoV
— 今村咲 (@saki_imamura) January 7, 2022
Pfizer Covid vaccine protects adolescents against multisystem inflammatory syndrome, CDC says https://t.co/85Nph22MBO
— CNBC (@CNBC) January 7, 2022
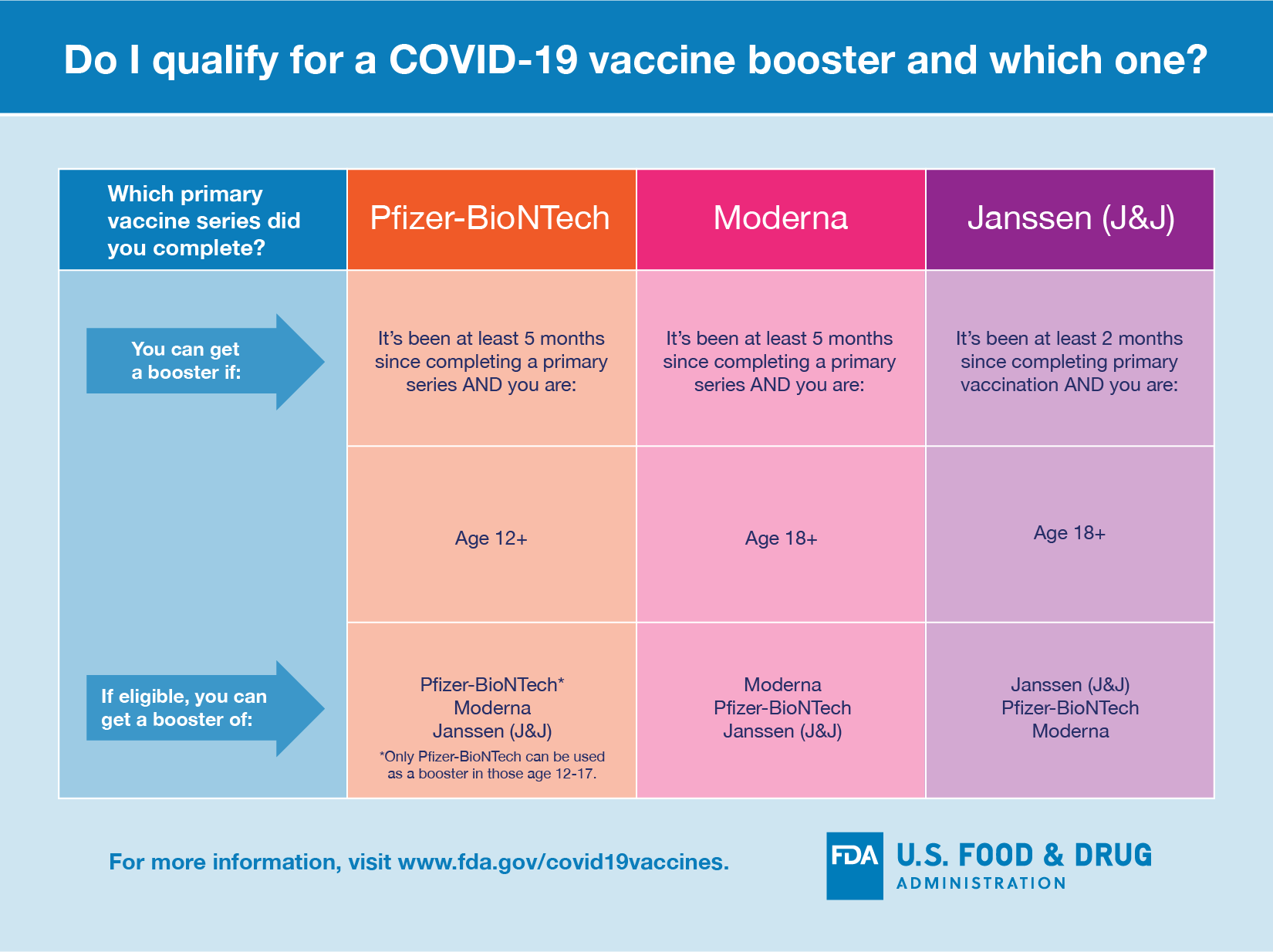
FDA shortens Moderna booster waiting period to 5 months for adults https://t.co/1qRphxRPbo
— CNBC (@CNBC) January 7, 2022
FDAがModernaのブースター接種も2回目接種から5ヶ月後に短縮したそう。https://t.co/Lfi3IRqyUJ https://t.co/3vkR0rUX7M
— 今村咲 (@saki_imamura) January 7, 2022
BREAKING
— C. Michael Gibson MD (@CMichaelGibson) January 7, 2022
Pfizer vax 91% effective in preventing Multisystem Inflammatory Syndrome in Children (MIS-C). MIS-C causes inflammation in children in organs including the heart, lungs, kidneys and brain two to six weeks after a mild or asymptomatic infection. https://t.co/H5uvCzzbor
Today, we amended the EUA for the Moderna COVID-19 Vaccine to shorten the time between the completion of a primary series of the vaccine and a booster dose to at least five months for individuals 18 years of age and older. https://t.co/xe3StVm0Zt pic.twitter.com/h6kd0Vty2P
— U.S. FDA (@US_FDA) January 7, 2022
??FDA Shortens Interval for Booster Dose of Moderna COVID-19 Vaccine to Five Months for individuals 18 years of age and older?? https://t.co/BhxGTqiNaV pic.twitter.com/m6mGPNQTwb
— Antibiotic Stewa®️x? Bassam Ghanem (@ABsteward) January 7, 2022
FDA Shortens Interval of Moderna COVID-19 booster to 5 Months.
— Priya Sampathkumar (@PSampathkumarMD) January 7, 2022
Pfizer reccs dropped 4 days ago
Do the @US_FDA and @CDCgov work hard at increasing confusion or does it just come naturally to them?
I feel like it’s 2020 all over again
https://t.co/Mc2poStOVc
PR is out: https://t.co/Q4dJKSbg1g
— David Lim (@davidalim) January 7, 2022
Everyone who tells you there is a covid "vaccine" is lying. The CDC changed the 100-yr old definition of "vaccine" to eliminate the requirement for the mRNA injections to actually immunize in order to accommodate these lies.https://t.co/pCsVB6Dg1W
— Dr. Stanch 24/7 (@Stanch247) January 7, 2022
@US_FDA Today amended the EUA for the Moderna COVID-19 Vaccine to shorten the time between the completion of a primary series of the vaccine and a booster dose to at least five months for individuals 18 years of age and older. https://t.co/CN2qg2kiic
— Medical Science and Technology #earlytreatments (@MedicalScitech) January 7, 2022
.@CDCgov found the Pfizer vaccine was 91% effective at protecting adolescents from multisystem inflammatory syndrome, or MIS-C. MIS-C is strongly linked to Covid infection and afflicts the heart, lung, kidneys, brain, skin, eyes or gastrointestinal organs.https://t.co/WgLvAhdsN5
— Ian Weissman, DO (@DrIanWeissman) January 8, 2022
FDA Shortens Interval for Booster Dose of Moderna COVID-19 Vaccine to Five Months https://t.co/XsOGs8kpUg
— Dr. Syra Madad (@syramadad) January 8, 2022
.png)